Full support and care
Regulatory Affairs
Technical documentation
SMQ
Audit
ISO 13 485 certification and CE marking
Quality assurance and regulatory affairs
Centaur clinical CRO has a panel of experts to support and/or take charge of the device’s technical file in accordance with European regulations (RDM 2017 / 745 – 746) and the company’s quality management system (QMS) in accordance with ISO 13 485.
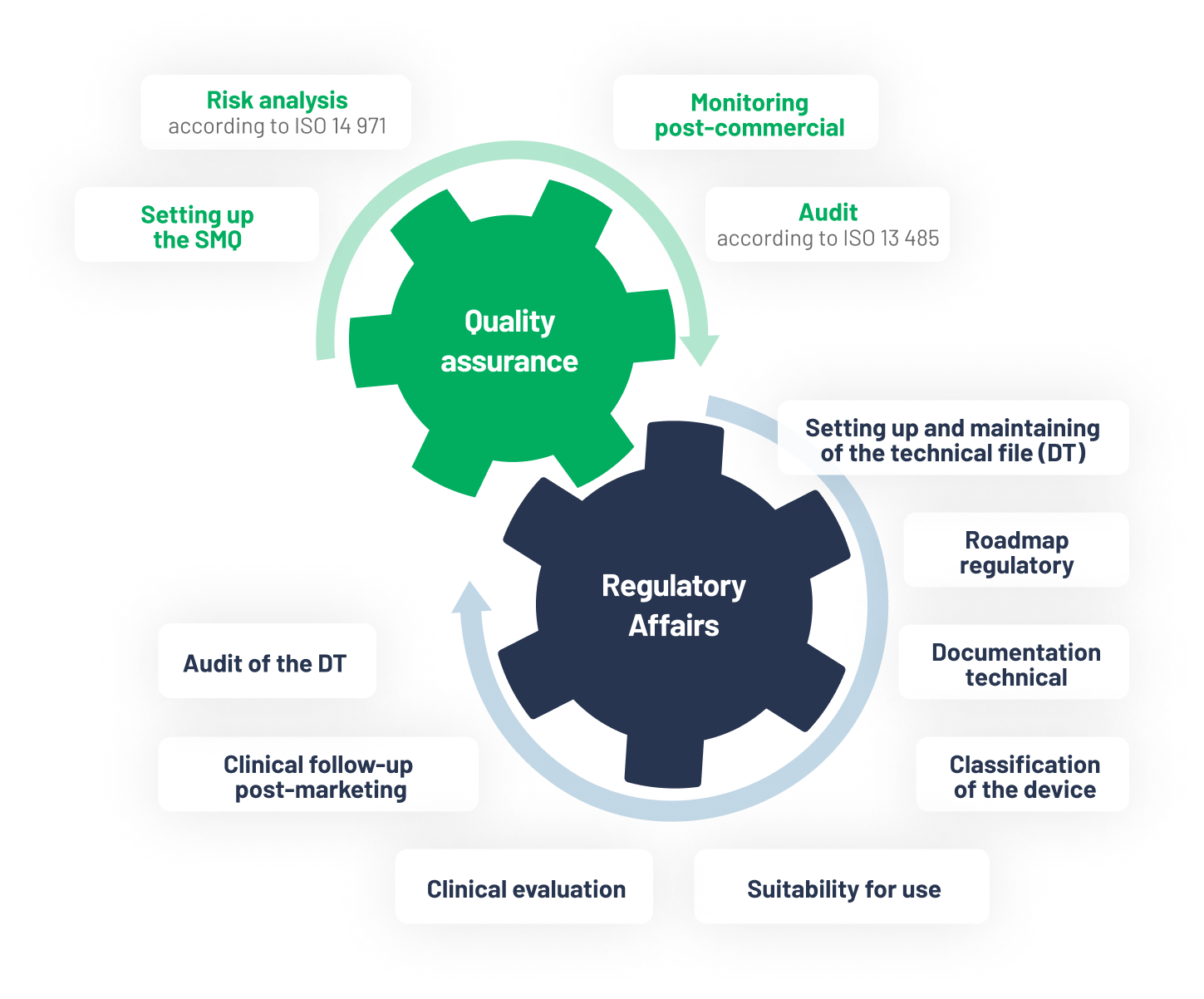
Centaur Clinical intervenes at any stage of the DT and QMS.
We support the manufacturer during the CE certification process with the notified body.
To find out everything you need to know about Regulatory Affairs, download our white paper in .pdf format.
Call on our team of experts for dedicated, optimised support
Because time is your ally, take advantage of our expertise and experience to bring your medical device to market.
TESTIMONIALS
Our satisfied customers are talking about us
We are starting a clinical investigation with Centaur Clinical and are very satisfied with our collaboration so far. Preparing the submission to the authorities is a stressful and time-consuming period that requires skilled staff. The Centaur Clinical teams are always ready to provide us with their expertise, availability and support.

Centaur Clinical helped us to define a rationale and a solid, coherent clinical investigation strategy in line with the expectations of Notified Bodies regarding the application of the MDR and the necessary clinical evidence to provide.
