Looking for a CRO in France?
Created in 2016, Centaur Clinical CRO supports manufacturers in the CE marking of their medical devices and IVDDs from preclinical testing, clinical trials, to post-marketing follow-up in Europe directly or with the collaboration of various partners.
Marking
The Centaur Clinical CRO team helps manufacturers of medical devices to obtain CE marking. Centaur Clinical CRO’s experience in conducting tests on medical devices has made it one of the leading references in Europe.
Centaur Clinical CRO relies on a network of complementary partners to offer medical device manufacturers turnkey CE marking.
Centaur Clinical CRO drafts regulatory documents and submits them to notified bodies and regulatory agencies/ethics committees.
A 140 m² laboratory with modern, secure equipment for your tests and trials.
Our expertise in submitting regulatory dossiers and conducting tests and trials enables us to offer you a relevant analysis that complies with the MEDDEV 2.7 rev. 4 guidelines and facilitates validation by the notifying body.
Centaur Clinical CRO has a quality management system and has achieved ISO 13485: 2016 certification in July 2021. CC services are conducted in accordance with ISO 14155, GLP and GCP.


Meet some of our team
A multilingual team on a human scale for dedicated, optimised support. Based in the south of France, Centaur Clinical has all the key skills required to support you throughout the CE marking process.
The preclinical team

Hassiba Mezrag
Scientific Director, Director of Regulatory Affairs & Customer Quality Affairs Director

Aurélie Da Silva
Head of Preclinical Trials

Lindsay Ramassy
Assistant Engineer
The clinical team

Elisa Faggianelli
Quality/CTM Manager

Luce Faedda
Clinical Research Associate and Data Manager
The regulatory team

Hassiba Mezrag
Scientific Director, Director of Regulatory Affairs & Customer Quality Affairs Director
Regulatory Affairs/ Quality Manager
The administrative and sales team

Gilda Iannella
Sales support/ADV

Corinne Ropa
Administrative manager
Our values
Centaur Clinical CRO has been committed to a responsible approach since its creation in 2016.
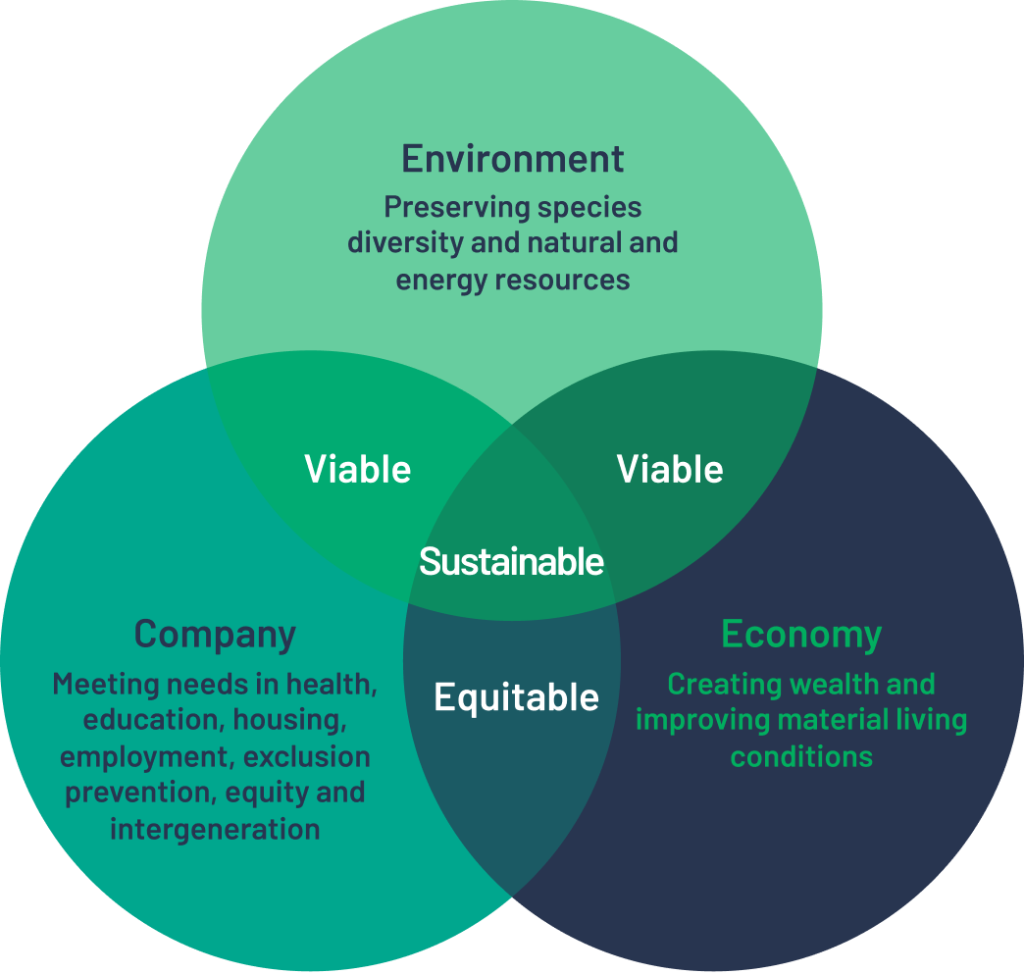
People and sustainable development are the company's core values.
To go further and formalise our commitments, we have signed up to the CEDRE PACA 2021-2023 programme. CEDRE is a support scheme run by the REGION SUD to promote ecological transition, support the circular economy and Corporate Social Responsibility (CSR).
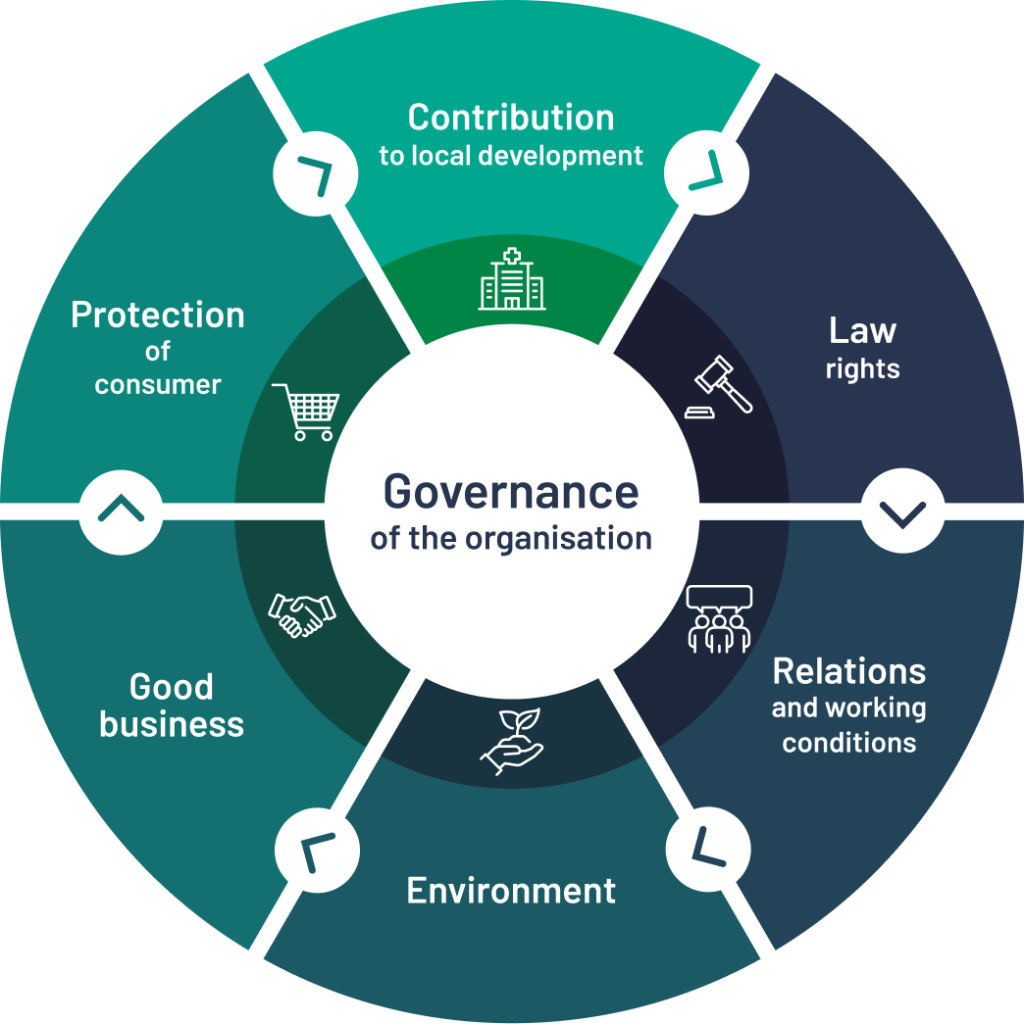
At Centaur, we place CSR at the heart of our challenges. To do this, we refer to ISO 26000, an international standard that defines the scope of CSR around seven central themes:
- Organisational governance
- Employment relations and conditions
- Fair practices
- Communities and local development
- Human rights
- Environment
- Consumer issues

Our CSR commitment
- Centaur Clinical CRO has signed a non-discrimination charter
- Centaur Clinical CRO is handi-friendly
- Centaur Clinical CRO adopts ethical and sustainable practices in the way it operates
- Centaur Clinical CRO has signed a gender pay parity charter
- At Centaur Clinical CRO, women account for more than 75% of employees
- Centaur Clinical CRO encourages work-life balance (flexible working hours and teleworking)
- Centaur Clinical CRO tries to select its suppliers based on criteria of proximity, eco-responsibility and CSR commitment
